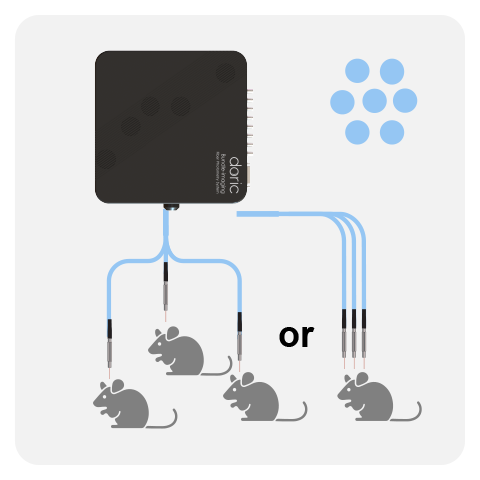
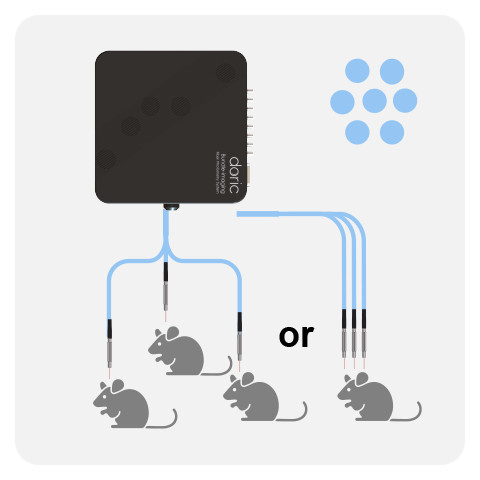
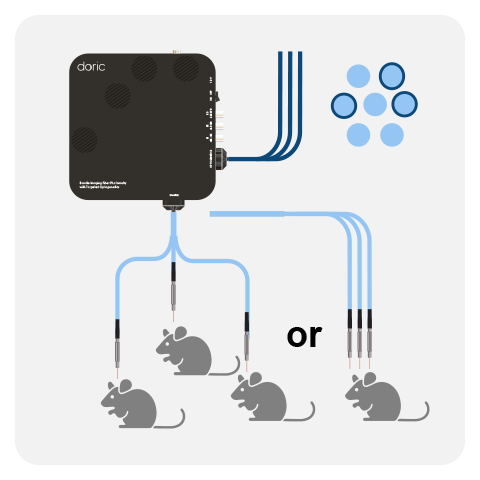
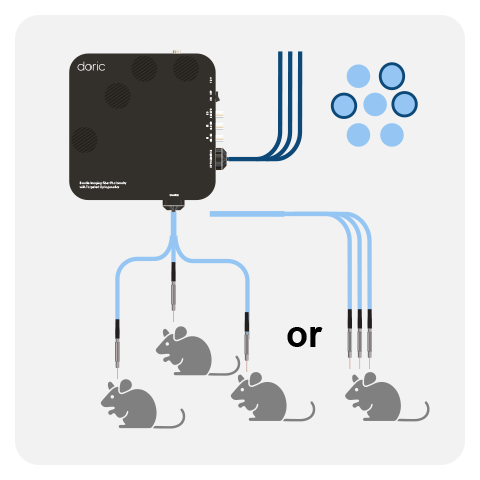
Fiber Photometry Solutions
Choose the right fiber photometry system for YOUR experimental needs.

All-in-one solutions
- Acquisition -> Data analysis
- Combine with behavior
- Compatible with optogenetics
- Plug-and-play
Fiber Photometry Systems
Fiber photometry is a neuroimaging technique that monitors neuronal activity in freely-moving animals. This technique utilises genetically encoded fluorescent indicators (e.g. GCaMP, dLight, GRAB-Ach, RCaMP, jRGECO1) expressed in target brain regions. These indicators fluorescence only when bound to melocules such as calcium, dopamine or acetylcholine, reporting real-time molecular dynamics during complex behaviors.
Unlike Doric Miniscopes, which resolve single-cell activity, fiber photometry records population signals (i.e bulk fluorescence from labelled neurons). But unlike microscopy, fiber photometry is minimally invasive, optimized for multi-animal & multi-site experiments, and more cost-effective.

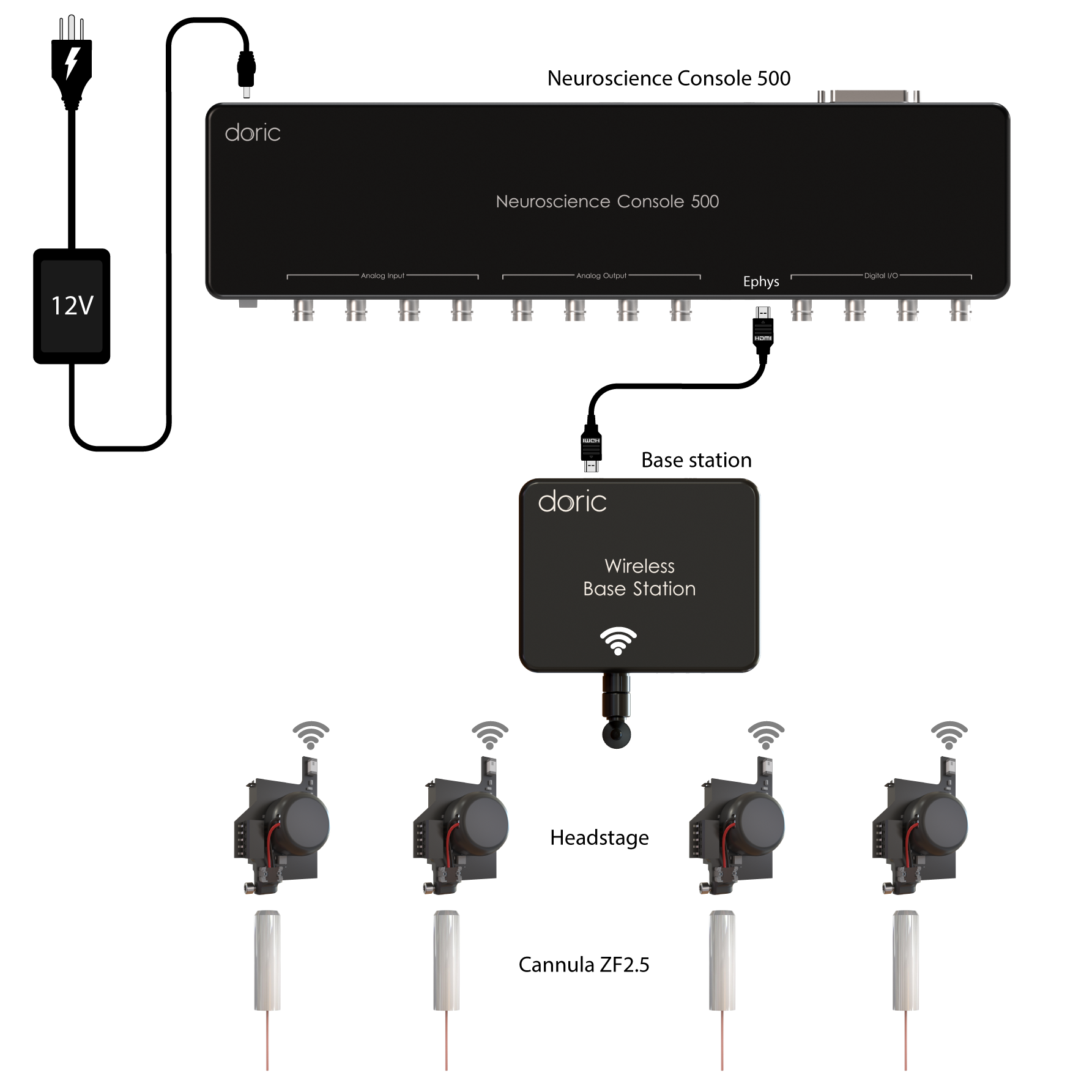
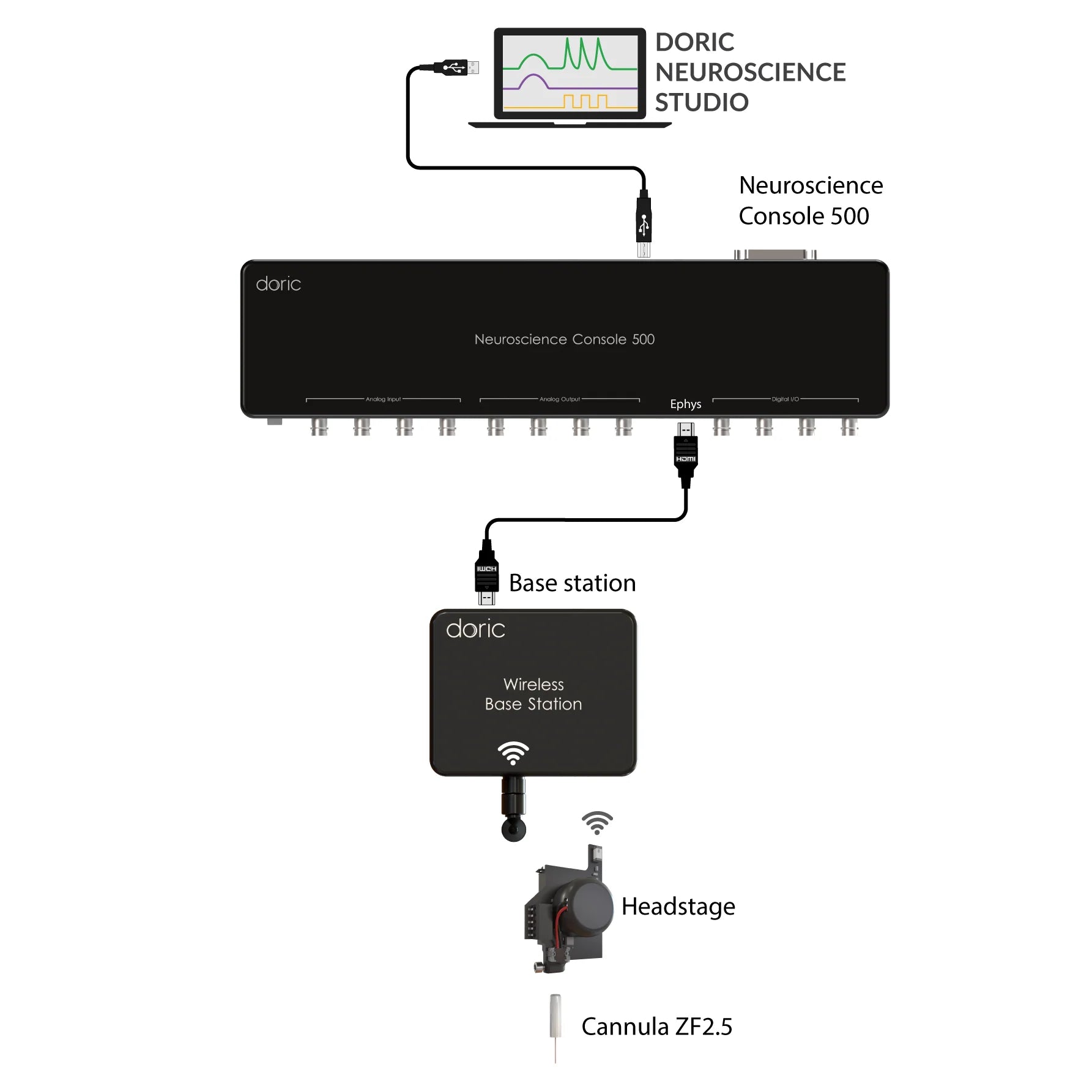
Fiber photometry system are composes of several core components that, together, combine multiple wavelengths into a single or mutliple fiber(s) and collect the emitted fluorescence response, including:
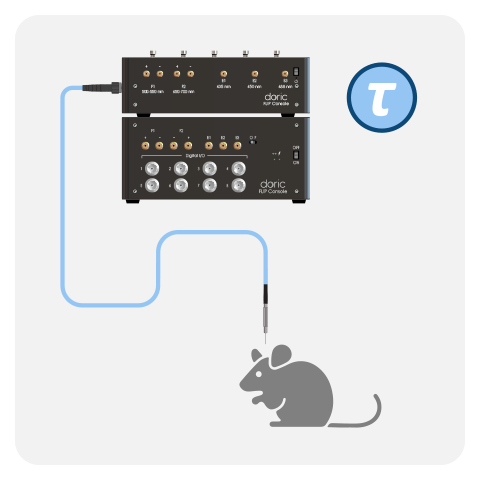
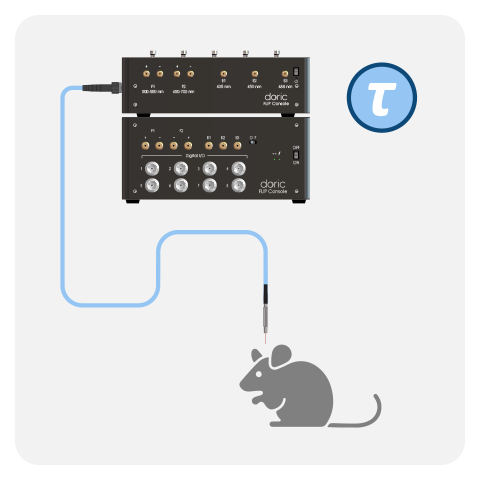
- Console
- LED Driver & LEDs
- Fluorescence mini cube (FMC)
- Fiber-optic patch cords
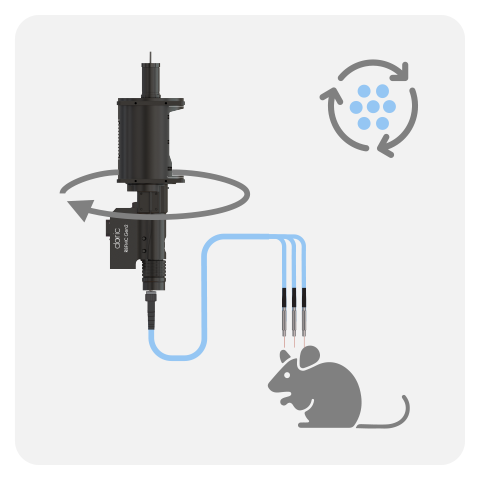
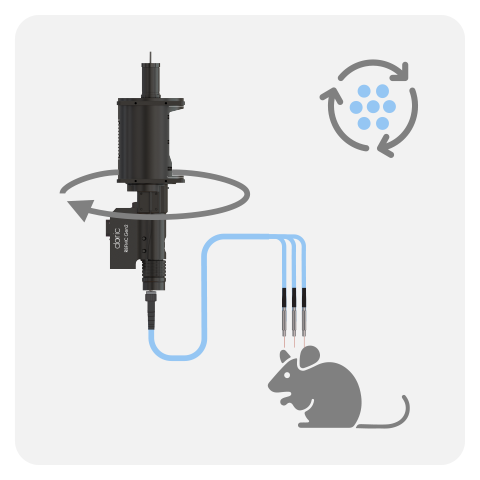
- Rotary Joints (commutators)
- Fiber-optic cannulas
- Data Acquisition & Analysis Softwares
The latest generations of Doric systems integrate multiple components into a single device, providing several advantages. Learn more.
Browse below the role of each fiber photometry component:
Doric Lenses Inc. is a recognized leader in advanced fiber photometry solutions for behaving animals, driving innovation in this rapidly evolving field. Each system was carefully engineered to address the specific experimental needs & challenges.
The three main types of Fibers Photometry Systems are split into categories based on the type of detector.
Explore how Doric’s fiber photometry systems compare across key metrics.
SYSTEM COMPARISON
This side-by-side comparison highlights differences in channel configuration, maximum number of site/animal(s), dual-color & optogenetics compatibility, and advanced features across seven distinct fiber photometry systems.
| BASIC | BUNDLE-IMAGING | LIFETIME | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
||
| Fiber Photometry |
Flexible isosbestic | ✔ | ✔ | n/a | ||||
|
Max # sites
●
◐
|
Up to 8 Up to 4 |
1–2 | 1 ✖ |
Up to 9–19* | Up to 9–19* | Up to 7–19* | 1–2 1 |
|
|
Multi-animal (separate cage)
●
◐
|
Up to 8 Up to 4 |
Up to 4 ✖ |
Up to 9–19* | Up to 9–19* | 1–2 ✖ |
|||
|
Multi-animal (same cage)
●
|
Up to 4 | |||||||
| Optogenetics | Same site as photometry | ✔ | ✔ | ✔*** | ✔ | |||
| Different site as photometry | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| Behavior (Rotary joint / commutator) |
Long-term, freely-moving behavior | ** | ✔ | 2–4 h | ** | ** | ✔ | ✔ |
| Integrated rotary joint | ✔ | n/a | ✔ | n/a | ||||
| Interacting animals | ✔ | |||||||
| Add Ephys / EEG & EMG | ** | ✔ | ** | ** | ✔ | ** | ||