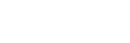Doric Miniscope Solutions
All-In-One Solution
What is miniscope?
Fluorescence Miniscope system images single-cell activity, in freely-behaving animals. Unlike two-photon and confocal microscopy, which requires head-fixation or anesthesia, miniaturized microscopy enables studying unrestrained behavior by directly attaching a small, lightweight microscope to the animal’s head. This technique utilizes genetically encoded fluorophore indicators, such as GCaMP, dLight, and GRAB-Ach, expressed in the target brain region. These indicators fluoresce when they bind specific molecules (e.g., dopamine, acetylcholine, calcium ions), serving as a proxy for neuronal activity. Thus, the miniaturized microscope is the ideal method to study the neuronal correlates of complex behaviors, in real time.

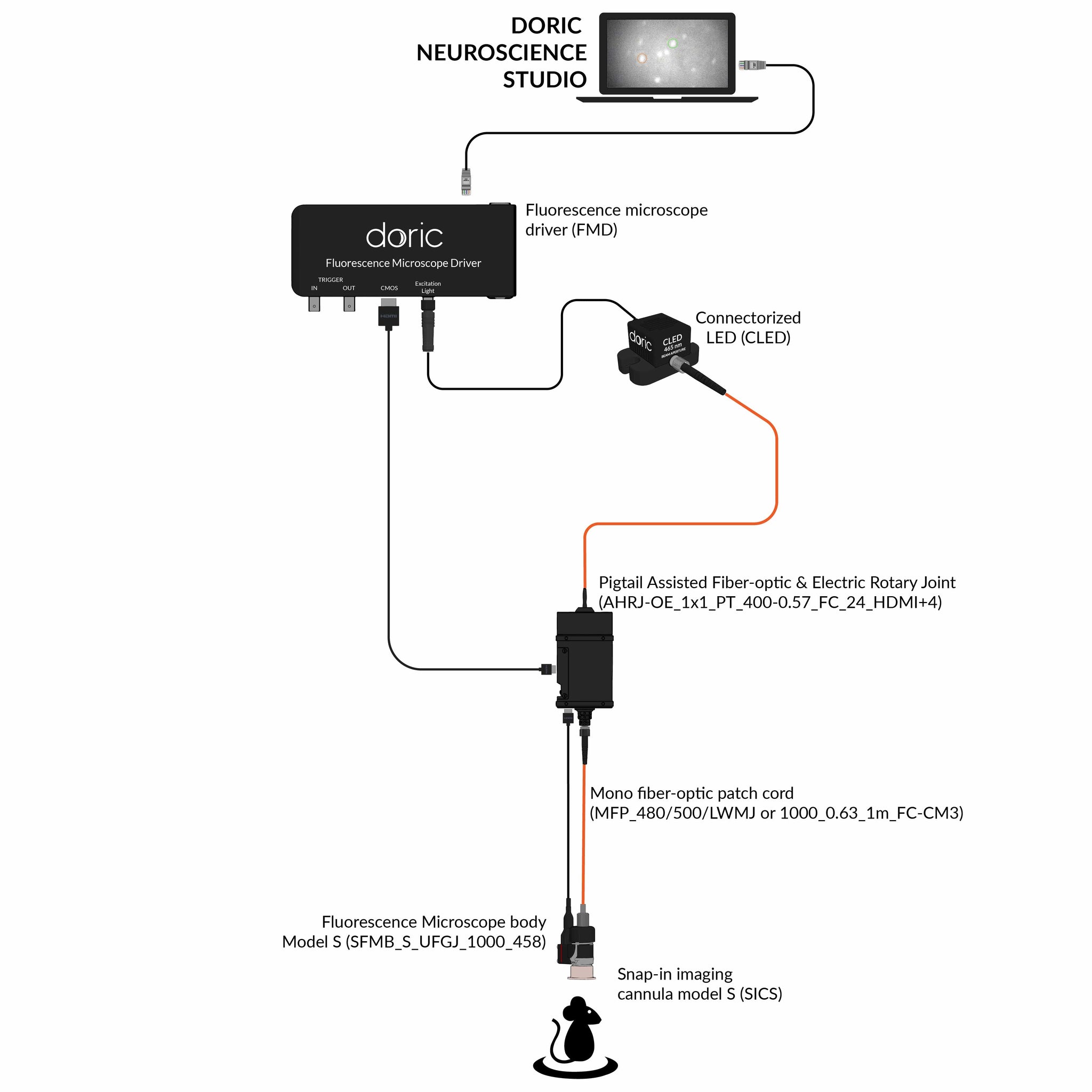
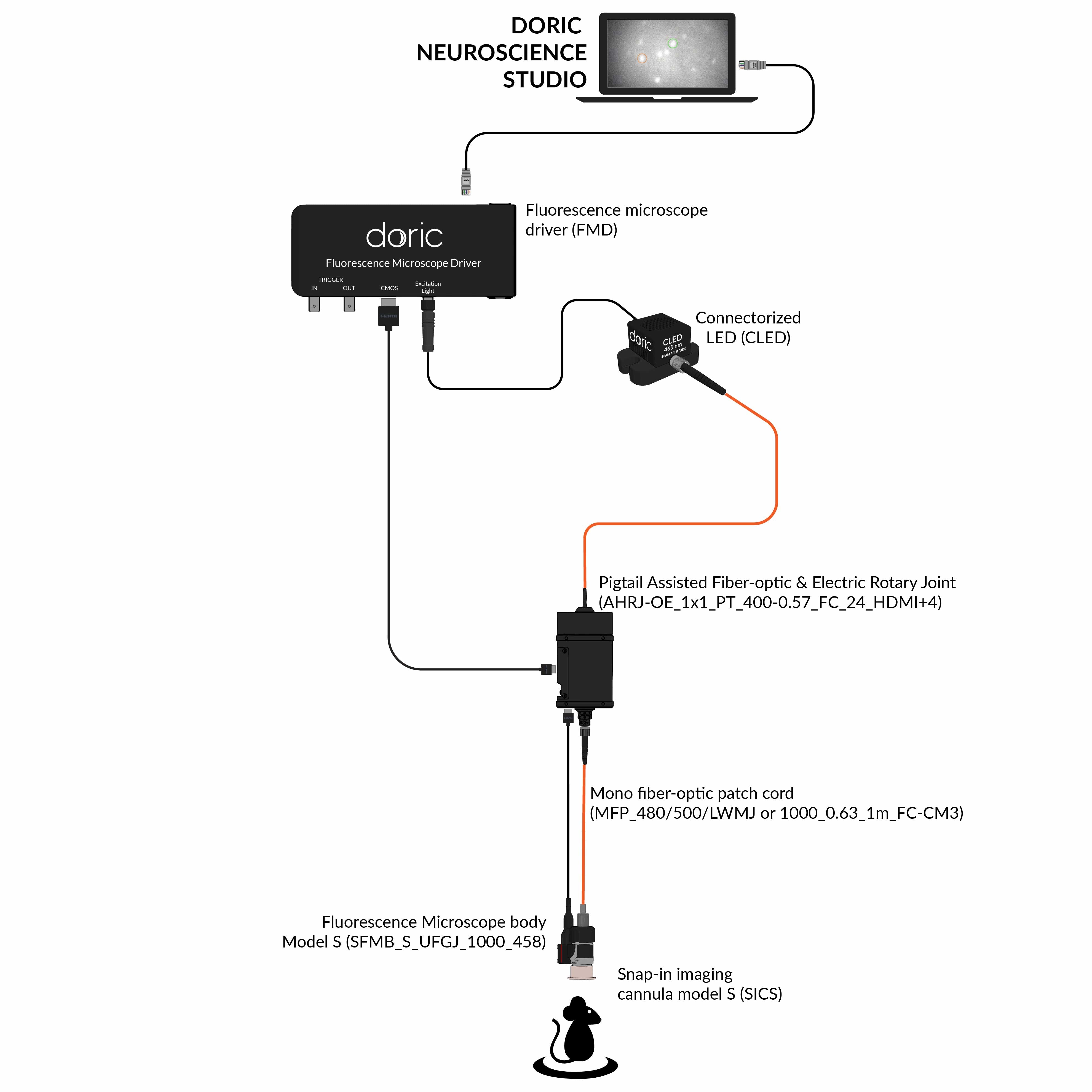
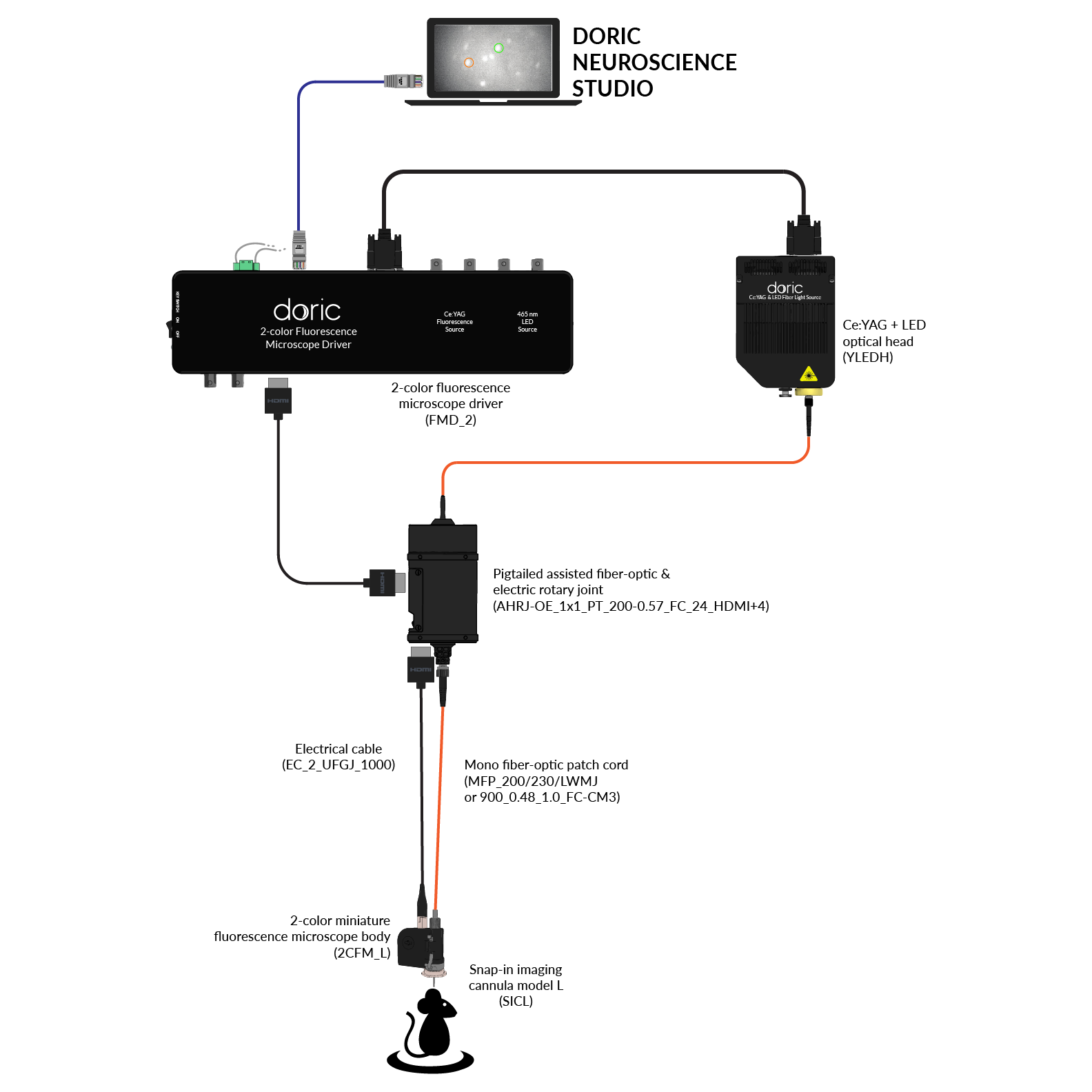
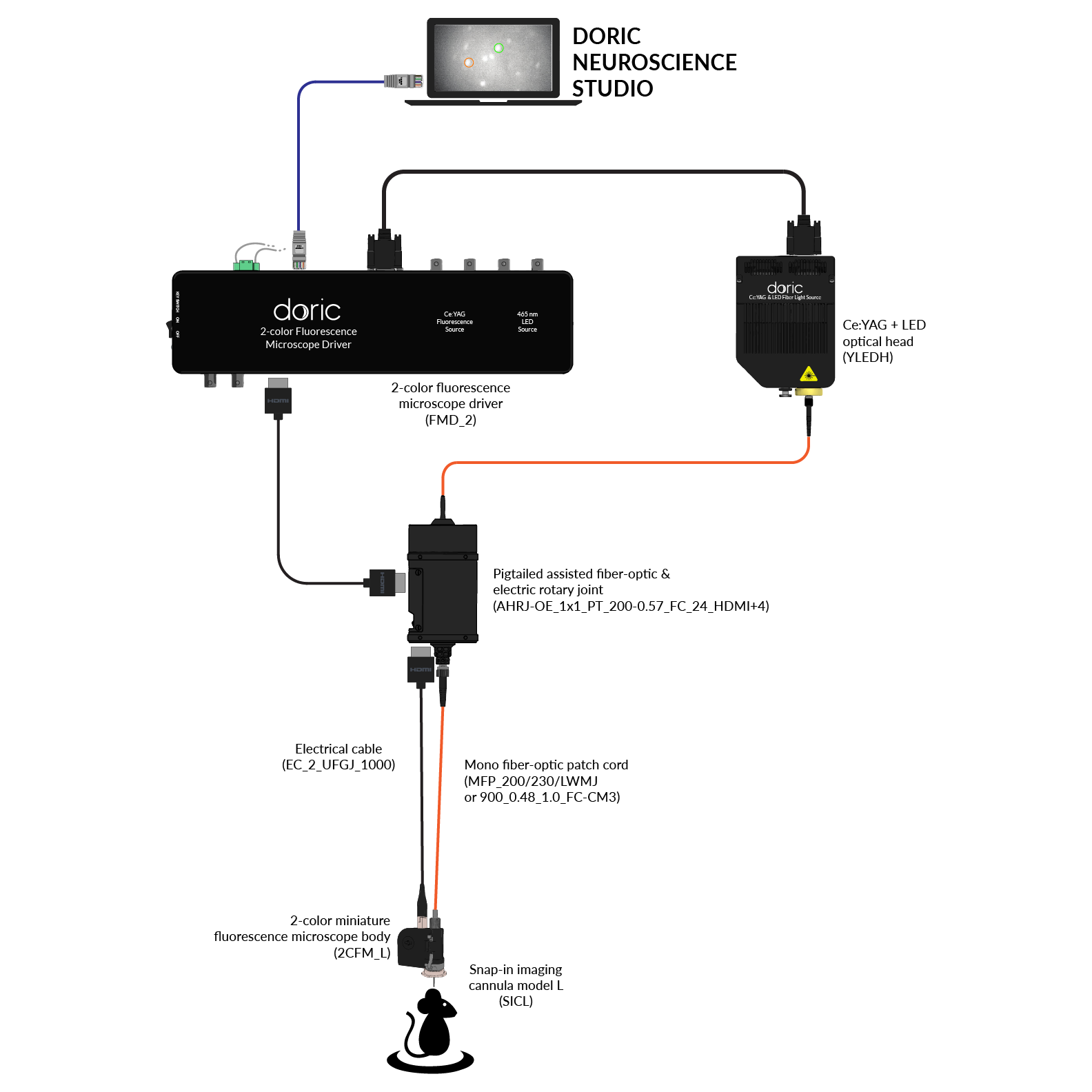
Since 2014, Doric Lenses has been among the leaders in the development of fluorescence miniscope systems and provides a comprehensive, all-in-one solution: from the implant to data analysis. This includes imaging cannulas, microscope body, rotary joint (commutator), Fluorescence Microscope Driver, and data analysis solution (danse™). The Doric miniscope systems are compatible with optogenetics and behavior acquisition (Behavior Camera/CamLoop), ensuring a seamless workflow.
As of today, several types of microscope bodies are carefully developed at the company to provide the best imaging quality based on the depth of the targeted brain structure. In this regard, Doric miniscopes can generally be categorized into two distinct sets:
• Surface Miniscope – Specifically designed for imaging superficial brain structures including all cortical areas.
• Deep Brain Miniscope – Optimized for all depth imaging including the deep brain structures, with the flexibility to also be used for surface area imaging.
See image below, which categorizes all Doric fluorescence miniscope systems:

Miniscope Options
Explore Additional Modalities
User-friendly data acquisition software

Doric Neuroscience Studio
FREE data acquisition software
Intuitive software with multiple modalities for controling all doric devices, from minisope to photometry, behavior and optogenetics.
All-in-one data analysis software
danse™ - data analysis solution
Automate Find Cell with integrated CaImAn, MiniAn, or Suit2p and streamline your parameter selection with multiple preview steps, without any coding required!Align cell traces with animal behavior, for batch processing all in one software.