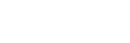Optogenetics Solutions
Optogenetics is a technique that uses light to stimulate or inhibit neuronal activity of genetically modified, light-sensitive neurons. Doric Lenses offers a wide range of vertically integrated products for optogenetics light delivery for in-vitro, acute or freely moving animals experiment. They include light sources, connecting fiber-optic patch cords, beamsplitters, fiber-optic rotary-joints, and fiber-optic cannulas.
Light Source
The selection of appropriate light source for light delivery into biological tissue via an optical fiber depends on the following parameters :
- wavelength [nm]
- optical power [mW] or intensity at fiber tip [mW/mm²]
- light intensity modulation capabilities
- power stability
Light source wavelength range should be adapted to the given opsin's action spectra. One of the most commonly used opsin is channelrhodopsin (ChR2) that has a good photocurrent response from 430 to 490 nm with a peak around 470 nm.
Other aspects to consider when selecting the light source and other parts of the setup are:
- optical fiber size or target area(s)
- number of sites: unilateral, bilateral or multiple sites
- common or independent control for multiple sites
- a chronic implant or acute probe
- experiment with freely moving animal may require fiber-optic rotary-joints
Experiments could combine different opsins having different spectral response, or fluorescent markers requiring multiple light sources and beam combiners. Our customer support can help you select the best-suited optical hardware for your optogenetics experiments.
Fiber-Optic Cannulas (or probe tip)
To optimize the light delivery into biological tissue, the optical fiber core diameter and numerical aperture have to be carefully selected. The larger optical fiber core diameter brings light to a larger area while making more damage to the tissue. A higher numerical aperture will spread light to a larger cone angle and will transmit more power when working with an incoherent light source as LED, but high numerical aperture optical fibers are in general not as robust as lower numerical aperture version due to difference in fiber construction.
The choice of optical fiber connector, or cannula receptacle, depends on the type of experiment and selected animal model. The simplest and the most common connection is the ferrule/sleeve with 1.25 or 2.5 mm diameter. Because of the smallest footprint and relatively good connection. However, the connecting requires some skill and can excerpt non-negligible pressure on the animal. Alternative solutions are offered, for example, if the experiment requires multiple connections and disconnections, the magnetic connector could be a solution. If the experiment involves larger and more active animals, the M3 screw-type connector provides a secure and reliable connection.
Lastly, the shape of the optical fiber tip may help to optimize the optogenetics illumination distribution or minimize the collateral tissue damage. Here is a short list of available fiber tip:
- Cleaved: A cleaved tip is the most economical solution, but not very precise or repeatable.
- Flat Tip (FLT): Flat tip termination provides a polished fiber tip and accurate protrusion length. When implanted in the brain it delivers a narrow cone of light in front of the fiber.
- Angle (A): Angle termination involves the polishing of the fiber tip at a chosen angle. This allows easier fiber penetration into tissue. When immersed into a high refractive index and highly scattering biological tissues, the light intensity distribution is almost the same as the flat tip.
- Cone (C): Cone termination involves the polishing of the fiber tip into a cone of outer angle. This allows easier fiber penetration into tissue. When immersed into a high refractive index and highly scattering biological tissues, the light intensity distribution is almost the same as the flat tip.
- Angle Mirror (MA): Angle mirror termination involves the polishing of the fiber tip at a chosen angle, typically 45 degrees with a mirror coating added on the polished surface to redirect light sideway.
- Tapered: Tapered fibers are pulled to give a sharp and narrow tip that facilitates penetration into tissue and reduces damage. Light output from the tapered tip is escaping all along the taper, as light refraction angle exceeds critical angle and is no more guided along the fiber. This can be used to illuminate a larger volume and avoid high light intensity localized at the fiber tip.
- Diffuser (DFL): Diffuser termination involves the addition of a diffusing material at the tip of the fiber. This increases the angular spread of light output from fiber compared to the relatively small output beam angle of a flat tip and helps to illuminate more neurons of interest for optogenetics or electrophysiology experiments.
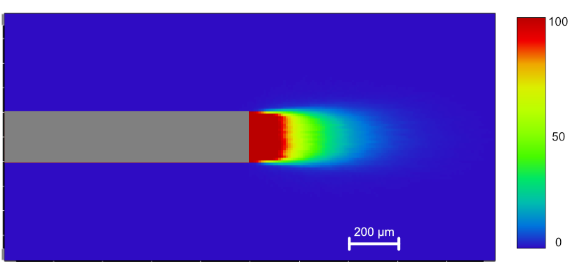
The light propagation into brain tissue will vary with the optical fiber type and the light source parameters. It can be visualized in this Application Note (the French version is available here).
External References
|
1. Separate anterior paraventricular thalamus projections differentially regulate sensory and affective aspects of pain. |
|
1. Noemi Rook et al. AAV1 is the optimal viral vector for optogenetic experiments in pigeons (Columba livia). Commun Biol.4, 100 (2021). Brain region: entopallium (important primary visual area in the pigeon) |
| 2. Fernández-García et al. M2 cortex-dorsolateral striatum stimulation reverses motor symptoms and synaptic deficits in Huntington’s disease. eLife 9, e57017 (2020). Brain region: cortico-striatal (M2-DLS) |
| 3. Brendan D. Hare et al. Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nature Communications 10, (2019). Brain region: medial prefrontal cortex (mPFC) |
|
4. Michelle M. Sidor et al. In vivo Optogenetic Stimulation of the Rodent Central Nervous System. J. Vis. Exp.95, e51483 (2015). Brain region: ventral tegmental area |
|
5. Gradinaru V et al.Targeting and readout strategies for fast optical neural control in vitro and in vivo. J Neurosci.27, 14231-8 (2007). |
| 6. addgene Optogenetics guide |
| 7. Deisseroth lab Optogenetics Ressource |